WHAT IS OZURDEX®?
- Diabetic macular edema (DME)
- Macular edema (swelling of the macula) following branch retinal vein occlusion or central retinal vein occlusion (RVO)
- Noninfectious uveitis (an inflammatory disease of the uvea) affecting the back segment of the eye

- A tiny corticosteroid implant that slowly releases medication over time, without the need for monthly injections. It will dissolve naturally and will not need to be removed
- Injected directly into the back of the eye, with minimal systemic absorption
OZURDEX® implant
size comparison.
- A tiny corticosteroid implant that slowly releases medication over time, without the need for monthly injections. It will dissolve naturally and will not need to be removed
- Injected directly into the back of the eye, with minimal systemic absorption
- The swelling in your retina can be caused by several factors, including inflammation
- OZURDEX® works to help reduce the inflammation in your retina and improve visual acuity
- Once injected, the implant dissolves slowly and releases a corticosteroid called dexamethasone
- Corticosteroids, such as dexamethasone, reduce inflammation
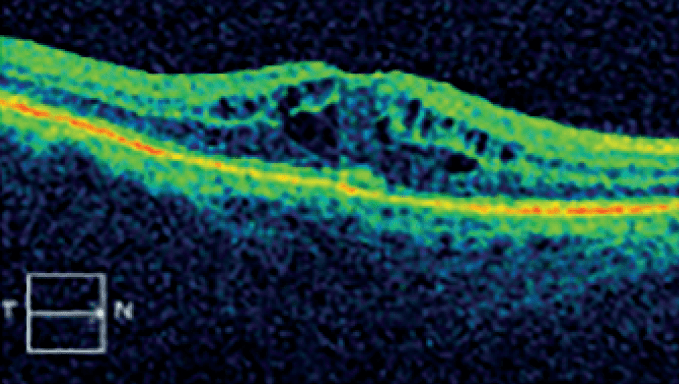
Optical coherence tomography (OCT) image of the retina of a person with DME.
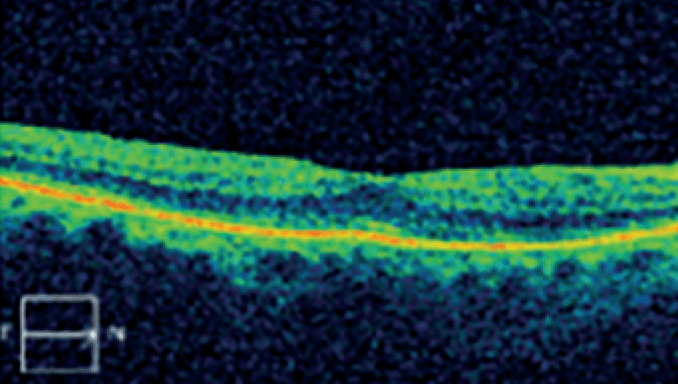
In clinical studies, OZURDEX® improved vision in patients without the need for monthly injections.
In clinical studies, OZURDEX® improved vision in patients without the need for monthly injections.
WHAT HAPPENS TO THE
OZURDEX® IMPLANT?
- The medication dissolves naturally over months
- The implant does not need to be removed


